|
| |
Phase-Change Media for CSP Thermal Energy Storage
We conduct our discussion of the potential difficulties that
accompany the use of PCMs as thermal storage media with a list of the basic
requirements that any potential PCM storage media must meet, as suggested by
Kenisarin (Kenisarin 2010):
-
Appropriate melting
temperature. The temperature must be within the operating range of
interest--usually comfortably within that range, so as to provide a buffer
for any transient effects (e.g., supercooling). Often times, appropriate
temperatures can be obtained by eutectic combinations of other salts, either
binary of ternary.
-
High specific heat, enthalpy of
fusion, and density. In other words, the PCM should have a high thermal
storage capacity, as would be expected of any successful candidate TES
medium.
-
Congruent phase change.
Congruent phase change, as opposed to incongruent phase change, results in
equal compositions of PCM constituents in both (solid and liquid) phases.
Contrast this with incongruent phase change, in which separate phases of
varied composition will appear at solidification--such is the case with
eutectics, although if their macrostructure remains relatively uniform
(e.g., lamellar microstructure), the effects of heterogeneity may be
negligible. Also, metal alloys may display incongruent melting, depending on
their composition.
-
Reliable convertibility after
repeated cycling. As may be the case with incongruent phase change, or if
phase change occurs in the presents of labile impurities, repeated thermal
cycling may compromise the thermophysical properties of the PCM. It has been
demonstrated that both the enthalpy of fusion and the melting temperature of
various eutectic salts and metal alloys can decline markedly after thermal
cycling on the order of 1000 times (Kenisarin 2010). Single-component salt
PCMs seem less prone to this problem.
-
High thermal conductivity. The
thermal conductivity will dictate the transient behavior of the storage
system, although convective effects will play a major role, as well
(Hernandez-Guerrero, Aceves et al. 1999). Unfortunately, most salts
considered in the context of PCMs display relatively low thermal
conductivities, on the order of 1 W/m-K (Kenisarin 2010). Use of adjuncts or
additives that improve the conductivity of these materials has been
investigated--specifically, graphite-salt composites show much improved
conductivity, while generally retaining desired phase change characteristics
(Pincemin, Olives et al. 2008). Container ribbing or incorporation of fins
are other possible means of improving heat transfer in poorly conductive
PCMs (Fernandes 2012). On the other hand, metal alloys may be a useful
substitute as high conductivity PCMs, thought they have their own
complications.
-
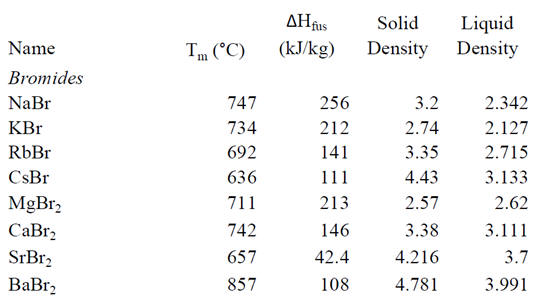
Tolerable volume change upon
phase change. Salts tend to have significant volume changes upon phase
change, and it is up to the designer to choose among candidate PCMs
appropriately and account for this effect. For illustration, we include a
table of bromide salts with the melting points and density changes upon
melting (Lide and Frederikse 1995). As can be seen, among these salts,
magnesium bromide appears the best suited for applications in which the
volume can change relatively little. Also, metal alloys typically have the
advantage of low volume change upon melting.
-
Insignificant supercooling.
Supercooling is the phenomenon whereby a system can cool to below its phase
change temperature without undergoing a phase change, usually the result of
a metastable state forming due to lack of nucleation points (Sandler 2006).
Such behavior is characteristic of crystalline salts, and can lead to rather
drastic transient effects (Kenisarin 2010). Here, again, metal alloys tend
to avoid this issue.
-
Chemical stability. The PCM
must remain chemically inert over the life of its use--ideally on the order
of many years.
-
Compatibility with construction
/ container / encapsulation materials. Material compatibility can be a
problem with salts especially, many of which have a high tendency to corrode
common construction materials such as iron. As such, extensive research has
been conducted investigating potential encapsulation techniques for use with
these PCMs (Zalba, Marin et al. 2003). Of course, metal alloys typically
will not cause corrosion, and so may be a worthwhile choice in this regard.
-
Tolerable toxicity. The
toxicity of these compounds warrants attention--the inclusion of adequate
health and safety measures in the manufacturing design may introduce
unforeseen costs.
-
Flame and fire safety. Again,
additional health and safety measures to contain the threat posed by
flammable or explosive candidate PCMs will likely introduce unforeseen
costs. Thus, these materials should be avoided.
-
Cost. Since the main point in
investigating PCMs is to reduce cost through improved efficiencies and
greater storage capacities, it is counter intuitive to select a
prohibitively expensive PCM, no matter how attractive its thermal transfer
properties may be. Hence, it is unlikely that materials incorporating rare
elements will find successful use in PCM storage systems. This criterion
also limits the applicability of many metal alloys.
Other issues related to the handling of PCM materials will
need to be addressed prior to manufacturing scale-up. For instance, the
hygroscopicity of many of the salts considered here will require rigorous care
to avoid infiltration of moisture into the encapsulated PCM matrix; in many
cases, the presence of moisture can drastically alter the phase change
temperature and overall chemical stability of the salt.
|
