|
Phase-Change Media for CSP Thermal Energy Storage
As described earlier, PCMs are characterized by a phase
transition at a temperature that falls within the range of their storage system
operation. Just as ice will melt in a glass of ice water as the ambient heat is
absorbed by it, so will a PCM, such as sodium chloride salt, melt as it absorbs
heat from the HTF that flows from the solar field. And the amount of heat
absorbed (or, alternately, released in the case of discharging) depends only on
the enthalpy change of fusion, which is essentially the same no matter which
direction the process runs (i.e., charge or discharge).
This characteristic immediately suggests two potential advantages to using PCMs
as the TES media: near isothermal heat transfer and increased volumetric storage
capacity.
Isothermal heat transfer seems somewhat unimportant, until you consider the
stresses that inhere on materials as they rapidly change temperature. Because
the PCM will charge (melt) or discharge (solidify) at one specific temperature,
this portion of the thermal storage cycle will represent a stagnation point on
the transient temperature curve and hence will reduce thermal stresses.
As for the volumetric storage capacity, we illustrate the potential for
improvement with a simple example (Goswami, Kreith et al. 2000). We compare
water, with a well known and relatively high specific heat capacity, with
Glauber's salt, which melts at approximately 32.4oC, over the range of
temperatures 30 to 60oC.
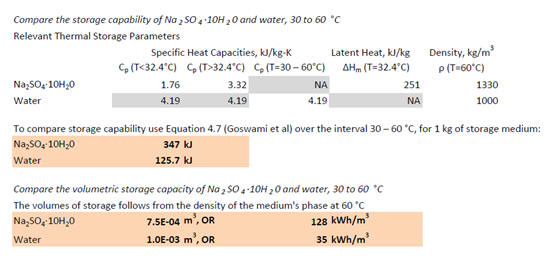
We see that over this temperature range, Glauber's salt holds
nearly three times the thermal energy as water, and it possesses an effective
volumetric storage capacity nearly four times that of water--and this is despite
its relatively low specific heat capacities in both the liquid and solid states.
This is the key to the potential value of PCMs as thermal storage media. If
appropriately chosen (i.e., relatively inexpensive salts with high enthalpies of
fusion and mass densities, with melting temperatures adequate for the operating
temperature range of interest), PCMs can greatly decrease the cost of thermal
energy storage. And, oftentimes, they are better suited for transfer at the
higher temperatures required for more efficient power block operation (e.g.,
solar tower plants). For instance, as reported by Herrmann et al, sodium
chloride, has a cost just slightly higher than the cheapest considered storage
media, concrete. Its melting point of 802oC, however, allows it to be considered
for substantially higher operating temperatures.
We next consider the potential difficulties one may encounter in the design of a
PCM TES system.
| 